Comparison ETFE VS ECTFE
Comparison ETFE VS ECTFE
Teflon® Finishes in the Chemical Processing Industry
A Comparison of ETFE and ECTFE Fluoropolymer Resins
INTRODUCTION
Fluoropolymers continue to find applications in a wide variety of chemical exposures due to their unique ability to resist chemical attack. Two types of coatings that are widely used are based on ETFE and ECTFE fluoropolymer resins.
- ETFE Ethylene – TetraFluoroEthylene
- ECTFE Ethylene – ChloroTriFluoroEthylene
Both of these resins fall into the category of “partially” fluorinated polymers because they contain ethylene as the comonomer. The incorporation of ethylene in the polymer changes the balance of properties compared to the “fully” fluorinated resins (PTFE, FEP and PFA). It imparts the very desirable property of “toughness,” as manifest by significantly better resistance to abrasion and mechanical damage, while sacrificing some chemical and temperature resistance. The net results, in general, are very tough, melt flowable polymers that can be applied in thick, chemically protective films with adequate temperature resistance to perform well in many industrial chemical processes.
Upon closer inspection, however, ETFE is found to have two distinct performance advantages over ECTFE: Higher temperature resistance and better chemical resistance. This paper explains the reasons for these advantages in terms of molecular composition and discusses additional benefits resulting from these two primary advantages.
FLUOROPOLYMER RESINS
There are a variety of fluorinated resins commercially available. Some examples follow, including the unfluorinated polyethylene polymer as a reference:
| Polyethylene (PE) | -(CH2 – CH2 – CH2 – CH2)- | Unfluorinated |
| Polyvinyl Fluoride (PVF) | -(CH2 – CHF – CH2 – CHF)- |  |
| Polyvinylidene Fluoride (PVDF) | -(CH2 – CF2 – CH2 – CF2)- | |
| Ethylene-Chlorotrifluoroethylene (ECTFE) | -(CH2 – CH2 – CF2 – CFCl)- | |
| Ethylene-Tetrafluoroethylene (ETFE) | -(CH2 – CH2 – CF2 – CF2)- | |
| Polytetrafluoroethylene (PTFE) | -(CF2 – CF2 – CF2 – CF2)- | Fully Fluorinated |
In general, as the fluorine content of the resin increases the polymer exhibits:
- Better Chemical Resistance
- Higher Operating Temperatures
- Better Nonstick
- Lower Combustibility
- Lower Coefficient of Friction
The chemical structures of ETFE and ECTFE are illustrated in Figures 1 and 2, which are molecular model representations that show only a short segment of these polymers. In these pictures, the C-C backbone of the polymer is difficult to see. It can be thought of as the central “core” of the polymer chain, around which the comparatively small hydrogen atoms (“white”), fluorine atoms (“green”) and large chlorine atoms (“black”) are attached.
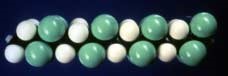
Figure 1: ETFE

Figure 2: ECTFE
Note that in ECTFE one chlorine atom has been substituted for a fluorine atom. As discussed later, this difference in molecular composition has a significant effect on performance properties.
FLUOROPOLYMER VARIATIONS
It is important to note that fluoropolymers are supplied in various “grades.” For example, Chemours supplies several different types of ETFE resins:
- For general purpose molding and extrusion uses.
- A high molecular weight grade for services requiring extra resistance to stress cracking or where more severe chemical resistance is needed.
- A lower molecular weight grade designed for high-speed extrusion in smooth, thin sections where physical property demands are minimal.
- Additional other grades for special processing needs.
ECTFE is also supplied in various grades for similar purposes.
Thus, not all ETFE and ECTFE polymers are created equal. For the purposes of this paper, “ETFE” as used here refers to the special grade that Chemours supplies for use as coatings for the Chemical Processing Industry (CPI). This particular ETFE resin has a compositional character that imparts higher temperature resistance and better chemical resistance than the broad spectrum of commercially available grades of ETFE mentioned above. Likewise, “ECTFE” as used here refers to the particular grade of polymer used for coatings.
GENERAL PROPERTY COMPARISONS
The commonality in molecular structures of ETFE and ECTFE could lead to the expectation that the performance properties are similar. However, the comparisons that follow, summarized from the test data from the supplier’s published literature, show significant differences as well as similarities.
Physical / Mechanical Properties
Both are tough polymers. They have similar nominal values for:
- Durometer Hardness
- Tensile Strength
- % Elongation
- Flexural Modulus
- Impact Strength
- Water Absorption
- Specific Gravity
- Coefficient of Friction
Both are good electrical insulators. They have similar nominal values for:
- Low Dielectric Constant High Dielectric Strength
- Low Dissipation Factors over a wide range of frequencies
- High Volume and Surface Resistivities
Both have high use temperatures. They have similar nominal values for:
- Maximum Service Temperature 150oC (300oF)
- Coefficient of Linear Thermal Expansion
- Deflection Temperature
- Thermal Conductivity
ETFE Advantages:
- Higher Melting Point, Higher Thermal Stability
- Lower Low Temperature Embrittlement
For the specific type of ETFE used for CPI coatings, Underwriter’s Laboratory (UL) has rated wire insulated with this material for service in appliances at a maximum continuous operating temperature of 200oC (392oF). This rating was determined under the guidelines of UL Subject 758 for appliance wiring material.
ETFE is More Thermally Stable than ECTFE
ETFE is affected by strong oxidizing acids, strong organic bases and sulfonic acids at elevated temperatures.ECTFE is affected by acids, bases and halogens at elevated temperatures, is attacked by amines, esters, and ketones, and is plasticized by halogenated solvents.
ETFE is More Chemically Resistant than ECTFE…
In Virtually All Classes of Compounds
At Higher Temperatures
POLYMER CHEMISTRY
One of the reasons ETFE coatings have better thermal stability and better chemical resistance than ECTFE, as noted previously, is due to the modified compositional character of this particular resin. The primary reason, however, is due to the presence of the chlorine atom in the ECTFE repeating polymer unit. As shown in the table below on bond strengths, the carbon-fluorine bond is significantly stronger than the carbonchlorine bond. Stronger atomic bonds are more difficult to break, either by chemical reaction or by thermal degradation. ETFE, therefore, is more chemically stable than ECTFE because it does not contain the weaker carbon-chlorine bond.
| BOND STRENGTH* | |
| ATOMIC PAIR | (Kcal / gram-bond) |
| C – F | 102 |
| C – H | 99 |
| C – C | 80 |
| C – Cl | 77 |
| * Gould, “Mechanism and Structure in Organic Chemistry,” 1959 | |
(Note that both polymers also contain the C-H bond, which is also weaker than the C-F bond. In fact, it is the presence of these weaker C-H bonds from the Ethylene monomer component that result in all partially fluorinated resins having lesser performance than the fully fluorinated resins).
Partially fluorinated fluoropolymers often react chemically by a mechanism called dehydrohalogenation. As shown for ETFE in Figure 3, dehydrohalogenation involves the simultaneous removal of a hydrogen atom and a fluorine atom from adjacent carbons in the C-C backbone. The two atoms then come off as hydrogen fluoride (HF), leaving a weaker double bond (C=C) in the polymer backbone. This double bond is relatively unstable and can subsequently more easily undergo further chemical attack, including scission of the polymer backbone.
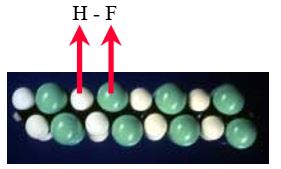
Figure 3: Dehydrohalogenation
Comparing ETFE with ECTFE in Figures 4 and 5, it is seen that the C-Cl bond strength is much weaker than that of C-F. Thus it takes less energy to remove a chlorine atom. Under the same circumstances, therefore, ECTFE is more likely to undergo dehydrohalogenation to form hydrogen chloride (HCl) than ETFE is to form hydrogen fluoride (HF). Furthermore, the weaker C-Cl bond is also more likely to be thermally broken, causing HCl evolution.
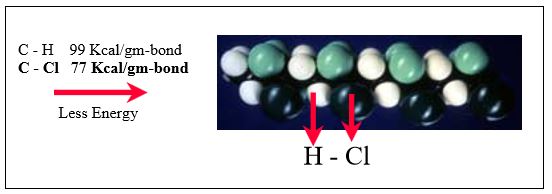
Figure 4: ECTFE contains a weaker carbon-chlorine bond
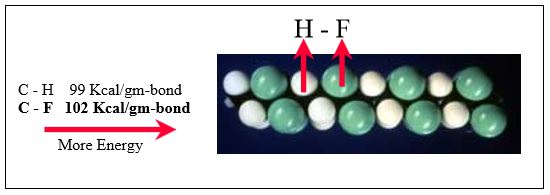
Figure 5: ETFE contains a stronger carbon-fluorine bond
Thus the better chemical resistance and higher thermal stability of ETFE, empirically observed in practice, is reinforced on the basis of sound chemical principles
ADDITIONAL COMPARISONS
Other factors that are often considered in deciding the choice of polymer for corrosive exposures include the following.
Melting Point – The melting point of ETFE, 245-250oC (473-482oF), is higher than ECTFE, 220oC (430oF). ETFE thus provides a higher margin of safety in the event of an accident (for example, a “runaway” chemical reaction that could develop temperatures much higher than normal).
Melt Flow Rate (MFR) – For a specific resin, Melt Flow Rate (ASTM D3159) is an indicator of molecular weight. The higher the molecular weight, the lower the melt flow rate. (Higher molecular weight resins are more viscous above their melting points and so they do not flow as well). Higher molecular weight polymers generally outperform their lower molecular weight analogs, especially having better stress crack resistance and better retention of properties after long term heat aging. For both processing and end use performance reasons, most fluoropolymers are supplied with melt flow rates in the range of 1 to 25. The ETFE resin used for ducts has an MFR of 6, which positions it in the medium to high molecular weight range.
Surface Smoothness – Cured films of ETFE are not quite as smooth as ECTFE, primarily due to its high molecular weight (low melt flow rate). In choosing the balance of resin properties use in broad chemical exposures, greater consideration was given to performance than to visual appearance. For the large majority of applications the surface smoothness of ETFE coatings is quite adequate.
Very smooth films may be important for certain specific applications, such as preventing bacterial growth in high purity deionized water delivery systems. For these special cases Chemours offers a second type of ETFE, used as a final topcoat, that provides an exceptionally smooth surface finish.
Permeation Resistance – ETFE and ECTFE are semi-crystalline polymers, meaning their films contain both crystalline and amorphous regions. ECTFE is claimed to be more crystalline than ETFE, which therefore imparts a lower permeation rate to certain gases and chemicals. However, permeation is affected by a number of factors, not only crystallinity. For example, ECTFE is more permeable to methylene chloride and methyl ethyl ketone due to its chemical similarity and molecular polarity, respectively.
In addition, permeation is inversely related to thickness. ETFE and ECTFE are applied in relatively thin films, in the range of 500 to 1,000 micrometers (20 to 40 mils). At these thickness levels, the rate of permeation can be significant for both polymers. Compare, for example, the sheet-lining thicknesses used for vessels in the Chemical Processing Industry, whose thicknesses of 1.5 to 2.3 mm (60-90 mils) thick are used. These very thick films significantly retard the rate of permeation, but do not stop it. Neither polymer is impermeable, even in very thick films (though at these very high thicknesses permeation can be reduced to commercial usefulness).
Furthermore, it would not be appropriate to base the performance of a coating system solely on the properties of the resin used. Neither resin, for example, adheres well by itself to metal substrates, and a primer coat is required. In the case of ETFE, the primer used contains other ingredients that provide excellent adhesion to various metals and also mitigates the effects of permeant chemicals. (For a more detailed treatment of this subject, please refer to the Chemours Fact Sheet entitled “PERMEATION – Its Effects on Teflon® Fluoropolymer Coatings”).
Flammability – By virtue of a laboratory test called the Limiting Oxygen Index (LOI), ECTFE is widely claimed to be less flammable than ETFE. LOI values are frequently presented to assert superiority of one resin over another in flammability. However, the LOI test was developed years ago by the National Aeronautics and Space Administration (NASA) to evaluate the combustion characteristics of materials used in the oxygen-rich environment inside space capsules. The test has little relevance beyond that, since the oxygen concentration in normal atmospheric air is only 21%. Except for such special cases, LOI values have yet to be shown relevant to end use performance in actual fire situations.
A more realistic test method is the FM-4922 Combustion test conducted by the Factory Mutual Research Corporation. This test is used to evaluate the combustibility of materials used for exhaust ducts in the Semiconductor Industry, and is the accepted Industry standard. ETFE and ECTFE coating systems both meet the rigorous requirements of this test and are “FM Approved.”
Processing – The method used to apply ETFE and ECTFE coatings is very similar. Both materials are applied (deposited) as dry powder coatings and subsequently baked to fuse the polymer into a cohesive film. Both utilize a primer first coat, followed by one or more topcoats (as needed) to achieve the desired final film thickness.
ECTFE is generally regarded as being easier to apply than ETFE. Its lower melting point, combined with its higher melt flow rate, allow this polymer to be cured to a smooth, glossy film using a bake oven temperature around 260oC / 500oF. However, ECTFE has a narrower “bake window” than ETFE, i.e., the time-temperature range within which it can be cured without being underbaked or overbaked. If allowed to remain in the bake oven too long, for example, ECTFE can begin to degrade (discoloration, blistering). This behavior requires close attention to controlling the baking/curing process, especially when parts having different sizes and masses are being coated.
ETFE, having a higher melting point and a lower melt flow rate, requires baking at 315oC (600oF). It is generally regarded as being more versatile from an overall processing standpoint. This is due to its exceptional thermal stability – its unique ability to withstand high temperatures for prolonged periods of time without sagging of the film or polymer degradation. Thus its “bake window” is much wider.
The bake window is an important fabrication parameter because it affects the quality of the final, cured coating. From a quality standpoint, it is essential that the coating on all parts – and all sections of all parts – be given the proper heat cure. This means that the temperature of the metal itself must be allowed to reach the recommended bake temperature, and then be allowed to dwell at that temperature for the recommended time. Only then will the coating achieve its maximum level of performance. This is critical. A proper bake is required to develop maximum coating integrity and adhesion to the metal substrate. This will avoid premature delamination problems after the part is put into service.
In practice, parts that are coated are seldom uniform in metal thickness. A typical vessel, for example, often has heavy metal flanges and other thick metal reinforcements welded to the (usually) thinner metal of the vessel wall. These flanges and reinforcements represent larger heat sinks; they heat up (and cool down) much slower. The effect of differential metal masses is shown schematically in Figure 6 (This diagram is for illustration purposes only – it does not represent an actual part).
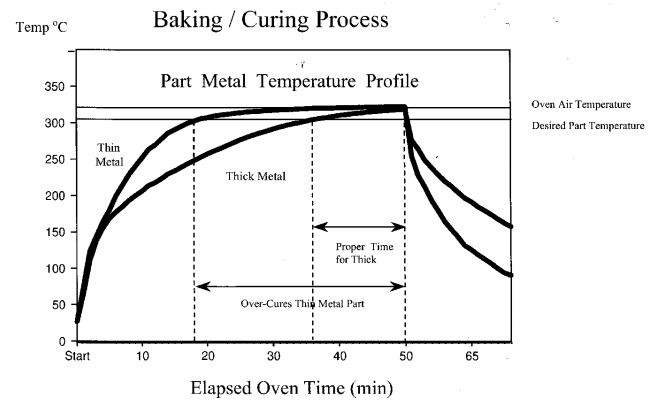
Figure 6: A proper bake is essential to good performance.
An undercured film will always fail prematurely.
Thick metal sections require a longer time to reach the proper bake temperature and must be allowed a sufficient dwell time at that temperature. In the meantime the thinner metal sections can become overbaked, leading to sagging or blistering of the coating or, in a severe case, thermal degradation of the polymer. Such coating defects can cause film discontinuities that are readily detected as “spark test” failures and result in reject parts. On the other hand, an undercure can only be detected by destructive testing. Only later, when the coating prematurely delaminates after exposure in service will this process deficiency become suspect.
The excellent thermal stability of ETFE eliminates this concern. ETFE coatings can tolerate prolonged, high-temperature bake times, thus assuring that the thick metal sections get properly cured without having to worry about the thin metal sections becoming overbaked. This wider operating window provides better assurance of good coating quality and greater reliably of the coating process, which results in fewer costly rejects.
SUMMARY
ETFE and ECTFE polymers are both used commercially as the base resins for thick film coatings used in the Chemical Processing Industry. ETFE has better chemical resistance and higher temperature resistance, as determined empirically and supported by a sound basis in chemical principles. These primary advantages not only provide an extra margin of performance in chemical service, but also contribute to a more reliable application process and improved quality of the final coating.
®registered trademark of Chemours, only available under license.



